Chemical Reactions and Stoichiometry
Interactive Video
•
Chemistry, Mathematics
•
10th - 12th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
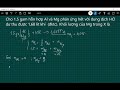
What is the total mass of the mixture of aluminum and magnesium used in the reaction?
2.5 grams
1.5 grams
2.0 grams
1.0 gram
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which gas is produced when aluminum and magnesium react with hydrochloric acid?
Carbon Dioxide
Oxygen
Nitrogen
Hydrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the valency of aluminum in the reaction?
1
2
3
4
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the valency of magnesium different from that of aluminum?
Magnesium has a valency of 4
Magnesium has a valency of 1
Magnesium has a valency of 2
Magnesium has a valency of 3
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the volume of hydrogen gas produced in the reaction?
1.68 liters
2.58 liters
2.68 liters
1.58 liters
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the number of moles of hydrogen gas?
Volume divided by 24
Volume multiplied by 22.4
Volume divided by 22.4
Volume multiplied by 24
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in setting up the system of equations for this problem?
Calculate the mass of hydrogen
Determine the moles of aluminum
Find the volume of magnesium
Establish the relationship between moles and mass
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Stoichiometry Concepts and Calculations
Interactive video
•
9th - 12th Grade

11 questions
Hydrochloric Acid Reaction Concepts
Interactive video
•
10th - 12th Grade

11 questions
Ionic Compounds and Concentration Calculations
Interactive video
•
9th - 12th Grade

11 questions
Molar Mass and Moles Calculations
Interactive video
•
9th - 12th Grade

11 questions
Oxidation-Reduction Reactions and Stoichiometry
Interactive video
•
10th - 12th Grade

11 questions
Limiting Reactants and Molar Mass
Interactive video
•
10th - 12th Grade

8 questions
IIT/JEE Chemistry Practice #2: Molar Mass/Stoichiometry
Interactive video
•
11th Grade - University

11 questions
Redox Reactions and Ionic Equations
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade