Understanding Bond Energy
Interactive Video
•
Chemistry, Science
•
9th - 12th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
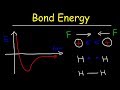
What happens to the energy of a system when two atoms are infinitely far apart?
The energy becomes positive.
The energy becomes zero.
The energy becomes infinite.
The energy becomes negative.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
At what point is the bond length of a molecule determined?
When the energy is positive.
When the energy is zero.
When the energy is at its minimum.
When the energy is at its maximum.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the energy of the system when atoms are extremely close to each other?
The energy decreases and becomes negative.
The energy becomes zero.
The energy remains constant.
The energy increases and becomes positive.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does the energy increase when atoms are very close to each other?
Due to electron-proton repulsion.
Due to proton-proton and electron-electron repulsion.
Due to electron-electron attraction.
Due to proton-proton attraction.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a positive energy value indicate about the forces between atoms?
The forces are balanced.
The forces are neutral.
The forces are repulsive.
The forces are attractive.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a negative energy value indicate about the forces between atoms?
The forces are attractive.
The forces are repulsive.
The forces are neutral.
The forces are balanced.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the hydrogen atom example, what type of bond is formed between the two hydrogen atoms?
Metallic bond
Non-polar covalent bond
Polar covalent bond
Ionic bond
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Molecular Geometry of N2O
Interactive video
•
9th - 10th Grade

11 questions
Nuclear Physics Concepts and Discoveries
Interactive video
•
9th - 12th Grade

10 questions
Potential Energy in Bond Formation
Interactive video
•
9th - 12th Grade

11 questions
Potential Energy and Bonding in Hydrogen Molecules
Interactive video
•
9th - 12th Grade

11 questions
Chemical Bonding and Energy Concepts
Interactive video
•
9th - 12th Grade

11 questions
Ionic Bonding Concepts and Lattice Energy
Interactive video
•
9th - 12th Grade

11 questions
Bond Energy and Potential Energy Concepts
Interactive video
•
11th - 12th Grade

11 questions
Molecular Geometry and Bond Angles
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

10 questions
Unit 1b Lesson 1 Quick Check
Quiz
•
9th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
12.2 Scientific Notation and Significant Figures
Quiz
•
10th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade