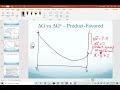
Understanding Delta G and Equilibrium
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary purpose of using graphs in the context of Delta G values?
To calculate the exact amount of reactants and products.
To determine the speed of a reaction.
To visually differentiate between non-standard and standard Delta G values.
To measure the exact value of Delta G in a lab setting.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When Delta G naught is negative, what does it indicate about the reaction at equilibrium?
The reaction is reactant-favored.
The reaction is non-spontaneous.
The reaction is product-favored.
The reaction is at a standstill.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the equilibrium constant (k) related to a product-favored reaction?
k is less than one.
k is equal to zero.
k is negative.
k is greater than one.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a negative slope of the tangent on a Delta G graph indicate?
The reaction is spontaneous.
The reaction is non-spontaneous.
The reaction is moving backward.
The reaction is at equilibrium.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
At what point on the graph does Delta G equal zero?
At the end of the reaction.
At the maximum point of the graph.
At the start of the reaction.
At the minimum point of the graph.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a reactant-favored system, where does the equilibrium lie on the graph?
Closer to the products.
At the maximum point.
At the midpoint.
Closer to the reactants.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of Delta G naught for a reactant-favored system?
Negative
Zero
Positive
Undefined
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Equilibrium Constant Calculations in Chemistry
Interactive video
•
11th - 12th Grade

11 questions
Understanding Delta G and Equilibrium
Interactive video
•
10th - 12th Grade

8 questions
Free Energy and the Equilibrium Constant
Interactive video
•
11th Grade - University

11 questions
Chemical Equilibrium Quiz
Interactive video
•
10th - 12th Grade

6 questions
Learning the Mechanisms : Investigating the Breakdown of Ammonia and Reaction Rates
Interactive video
•
10th Grade - University

11 questions
Delta G and Free Energy Concepts
Interactive video
•
9th - 12th Grade

11 questions
Acid-Base Equilibrium Concepts
Interactive video
•
11th - 12th Grade

11 questions
Standard Reaction Entropy Calculations
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade