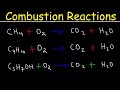
Balancing Combustion Reactions
Interactive Video
•
Chemistry, Science
•
9th - 12th Grade
•
Hard
Amelia Wright
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the typical products of a complete combustion reaction involving hydrocarbons?
Carbon dioxide and water
Hydrogen and carbon
Carbon monoxide and water
Methane and oxygen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a complete combustion reaction of methane, what is the first step in balancing the equation?
Balance the oxygen atoms
Balance the hydrogen atoms
Balance the carbon atoms
Balance the nitrogen atoms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When balancing the combustion of propane, how many carbon dioxide molecules are produced?
Four
Three
Two
One
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of oxygen atoms on the right side when balancing the combustion of propane?
Fourteen
Twelve
Ten
Eight
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the combustion of ethane, what is the coefficient of oxygen when the equation is balanced?
Three
Nine
Five
Seven
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you handle fractions when balancing combustion reactions?
Leave them as they are
Ignore them
Multiply all coefficients by two
Divide all coefficients by two
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing the combustion reaction of butane?
Balance the hydrogen atoms
Balance the nitrogen atoms
Balance the oxygen atoms
Balance the carbon atoms
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Balancing Equations Practice Challenge
Interactive video
•
9th - 12th Grade

11 questions
Understanding Theoretical and Percent Yield in Combustion Reactions
Interactive video
•
9th - 12th Grade

11 questions
Balancing Chemical Equations Concepts
Interactive video
•
10th - 12th Grade

9 questions
Balancing Chemical Equations
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 12th Grade

11 questions
Understanding Cellular Respiration and Photosynthesis
Interactive video
•
9th - 12th Grade

8 questions
The EASY way to balance Combustion Reactions!
Interactive video
•
10th Grade - University
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

10 questions
Unit 1b Lesson 1 Quick Check
Quiz
•
9th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
12.2 Scientific Notation and Significant Figures
Quiz
•
10th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade