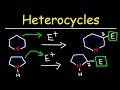
Electrophilic and Nucleophilic Aromatic Substitution
Interactive Video
•
Chemistry, Science
•
10th Grade - University
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does bromination of pyrrole not require a Lewis acid catalyst?
Pyrrole is less reactive than benzene.
Pyrrole is not an aromatic compound.
Pyrrole is more nucleophilic than benzene.
Bromine is a weak electrophile.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which position does bromine prefer to substitute in pyrrole?
Position 4
Position 3
Position 2
Position 1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is more nucleophilic than benzene?
Furan
All of the above
Thiophene
Pyrrole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is thiophene less nucleophilic than furan?
Furan has a larger ring size.
Thiophene is not aromatic.
Sulfur's 3p orbital does not overlap effectively with carbon's 2p orbital.
Sulfur is more electronegative than oxygen.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does electrophilic attack prefer the two position in pyrrole?
It allows for more resonance structures.
It is less sterically hindered.
It is closer to the nitrogen atom.
It is more electronegative.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In pyridine, which position is favored for electrophilic substitution?
Position 2
Position 3
Position 1
Position 4
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is pyridine less reactive than benzene in electrophilic aromatic substitution?
Pyridine is not aromatic.
Pyridine has a nitrogen atom that deactivates the ring.
Pyridine is more nucleophilic.
Pyridine has a larger ring size.
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

6 questions
Write the equation of a line parallel to another through a point
Interactive video
•
11th Grade - University

11 questions
Carbocation Stability and Effects
Interactive video
•
10th - 12th Grade

6 questions
The History and Making of Tea
Interactive video
•
KG - University

8 questions
Learn to solve a rational equation by factoring
Interactive video
•
11th Grade - University

8 questions
Learn How to Evaluate the Composition of Two Functions
Interactive video
•
11th Grade - University

6 questions
Pi-n Conjugation: Unlocking the Secrets of Molecular Stability
Interactive video
•
10th Grade - University

8 questions
Beckmann Rearrangement
Interactive video
•
11th Grade - University
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade