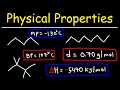
Understanding Alkanes: Properties and Stability
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following statements about the melting points of alkanes is correct?
All alkanes have the same melting point.
Shorter straight-chained alkanes have higher melting points.
Longer straight-chained alkanes have higher melting points.
Melting points are not affected by the chain length of alkanes.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary reason longer alkanes have higher melting points?
They have more ionic bonds.
They have more covalent bonds.
They have more Van Der Waal forces.
They have more hydrogen bonds.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the density of alkanes change with molecular weight?
Density remains constant regardless of molecular weight.
Density increases as molecular weight increases.
Density fluctuates randomly with molecular weight.
Density decreases as molecular weight increases.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which alkane property increases with molecular weight?
Boiling point
Density
Solubility in water
Color
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the relationship between the heat of combustion and the stability of alkanes?
Heat of combustion does not affect stability.
Stability is unrelated to heat of combustion.
Lower heat of combustion indicates higher stability.
Higher heat of combustion indicates higher stability.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a lower absolute value of heat of combustion indicate about an alkane?
It is less reactive.
It is more reactive.
It is more stable.
It is less stable.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do branched alkanes have lower boiling points than straight-chain alkanes?
They have higher molecular weights.
They have less area of contact between molecules.
They have more Van Der Waal interactions.
They are more polar.
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Properties and Behavior of Matter
Interactive video
•
9th - 12th Grade

11 questions
Addition Reactions of Alkenes
Interactive video
•
9th - 12th Grade

9 questions
Alkanes and Their Properties
Interactive video
•
9th - 10th Grade

11 questions
Boiling Points and Molecular Interactions
Interactive video
•
9th - 12th Grade

11 questions
Intermolecular Forces and Boiling Points
Interactive video
•
10th - 12th Grade

8 questions
Functional Isomerism & Metamerism: Discovering Molecular Twins
Interactive video
•
10th Grade - University

11 questions
Molecular Interactions and Boiling Points of Propane and Acetaldehyde
Interactive video
•
9th - 12th Grade

6 questions
How to Supercool Water: A SciShow Experiment
Interactive video
•
11th Grade - University
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade