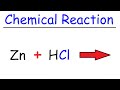
Zinc and Hydrochloric Acid Reactions
Interactive Video
•
Chemistry, Science
•
9th - 12th Grade
•
Practice Problem
•
Easy
Lucas Foster
Used 1+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of reaction occurs between zinc metal and hydrochloric acid?
Single replacement reaction
Decomposition reaction
Synthesis reaction
Double replacement reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of a zinc ion in the reaction with hydrochloric acid?
1-
1+
2-
2+
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What gas is produced when zinc reacts with hydrochloric acid?
Oxygen
Nitrogen
Chlorine
Hydrogen
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction, what happens to zinc?
It forms a precipitate
It remains unchanged
It is oxidized
It is reduced
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following metals cannot displace hydrogen from hydrochloric acid?
Zinc
Copper
Magnesium
Aluminum
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of consulting the activity series of metals?
To measure the temperature change in reactions
To determine the solubility of metals
To predict the color change in reactions
To check if a metal can displace another in a reaction
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing the chemical equation for the reaction?
Balance the oxygen atoms
Balance the chloride ions
Balance the zinc atoms
Balance the hydrogen atoms
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Fundamentals of Chemistry Concepts
Interactive video
•
9th - 12th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
10th - 12th Grade

8 questions
Italy Vs Uk: 10 Shocking Differences
Interactive video
•
KG - University

11 questions
Practical - Force and Acceleration
Interactive video
•
10th Grade - University

6 questions
How to Find a Humpback Whale
Interactive video
•
10th - 12th Grade

11 questions
Understanding Hess's Law and Enthalpy Changes
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

20 questions
Periodic Table & Trends
Quiz
•
10th Grade

17 questions
Reaction Rates
Quiz
•
11th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

24 questions
Identifying Types of Chemical Reactions
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade