Electrolysis Reactions and Concepts
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Practice Problem
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
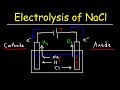
What type of electrodes are used in the electrolysis of sodium chloride solution?
Copper
Iron
Graphite
Zinc
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the electrolysis setup, which terminal is the anode connected to?
Negative terminal
Positive terminal
Both terminals
Neither terminal
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ions are attracted to the anode during electrolysis?
Protons
Neutral atoms
Cations
Anions
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the principal oxidation reaction at the anode in a concentrated NaCl solution?
Oxidation of water
Oxidation of chloride
Reduction of sodium
Reduction of hydrogen
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which reaction is more spontaneous at the anode in a standard one molar NaCl solution?
Reduction of hydrogen
Reduction of sodium
Oxidation of chloride
Oxidation of water
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the principal reduction reaction at the cathode in an acidic solution?
Reduction of sodium ions
Reduction of hydrogen ions
Oxidation of chloride ions
Oxidation of water
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a molten NaCl solution, what forms at the cathode?
Oxygen gas
Hydrogen gas
Sodium metal
Chlorine gas
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

7 questions
(Micro 1.1) Thinking Like an Economist - Scarcity
Interactive video
•
9th - 12th Grade

5 questions
F.5 Electrolysis
Interactive video
•
9th - 12th Grade

6 questions
VOICED: 'Sons of Iraq' feel betrayed by motherland
Interactive video
•
10th Grade - University

6 questions
BCA Protein Assay Quiz
Interactive video
•
10th - 12th Grade

11 questions
Understanding Greenwashing and Sustainability
Interactive video
•
9th - 12th Grade

11 questions
Fundamentals of Chemistry Concepts
Interactive video
•
9th - 12th Grade

6 questions
Federal Income Tax and Federal Reserve Quiz
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade

10 questions
Periodic Table Families and Groups
Quiz
•
10th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

22 questions
Solubility Curve Practice
Quiz
•
10th Grade