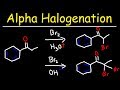
Alpha Halogenation of Ketones
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Ethan Morris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the major product when acetone reacts with bromine under acidic conditions?
Acetone with one bromine atom replacing an alpha hydrogen
Acetone with two bromine atoms replacing alpha hydrogens
Acetone with no bromine atoms
Acetone with a hydroxyl group
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in the mechanism of alpha halogenation under acidic conditions?
Protonation of the ketone by hydronium ion
Direct reaction with bromine
Formation of the enolate ion
Deprotonation of the alpha hydrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the acidic mechanism, what form does the ketone convert into before reacting with bromine?
Radical form
Enol form
Carbanion form
Enolate form
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the intermediate formed during the basic mechanism of alpha halogenation?
Enolate ion
Enol
Carbocation
Radical
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the enolate ion react with bromine in the basic mechanism?
By attacking the bromine molecule
By losing a proton
By forming a pi bond
By forming a radical
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the practice problem under acidic conditions, how many alpha hydrogens are replaced?
One
Two
None
All
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the major product when a ketone reacts with excess bromine under acidic conditions?
Ketone with one bromine atom
Ketone with two bromine atoms
Ketone with no bromine atoms
Ketone with a hydroxyl group
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

6 questions
Favorskii Rearrangement
Interactive video
•
11th Grade - University

5 questions
Organic Chemistry Synthesis Challenge 6
Interactive video
•
11th Grade - University

6 questions
What Happens When You Stop Eating?
Interactive video
•
11th Grade - University

8 questions
Introduction to Transition Metal Catalysis
Interactive video
•
11th Grade - University

8 questions
Khám Phá Hạt Nhân Nguyên Tử
Interactive video
•
9th - 12th Grade

11 questions
Alpha Decay and Isotope Stability
Interactive video
•
9th - 10th Grade

11 questions
Synthesis Reactions and Mechanisms
Interactive video
•
11th - 12th Grade

11 questions
Alpha Substitution Reactions Quiz
Interactive video
•
11th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade