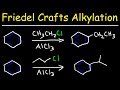
Friedel-Crafts Alkylation Concepts
Interactive Video
•
Chemistry, Science
•
10th Grade - University
•
Practice Problem
•
Hard
Ethan Morris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main purpose of using aluminum chloride in the Friedel-Crafts alkylation reaction?
To stabilize the benzene ring
To serve as a catalyst
To provide a source of chlorine
To act as a solvent
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the major product when benzene reacts with tert-butyl chloride in the presence of AlCl3?
Ethyl benzene
Propyl benzene
Isopropyl benzene
Tert-butyl benzene
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a limitation of the Friedel-Crafts alkylation reaction?
It only works with aromatic compounds
It can lead to polyalkylation
It requires high temperatures
It produces only one type of product
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following groups is a weakly activating group in Friedel-Crafts alkylation?
Amino group
Carboxyl group
Alkyl group
Nitro group
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the benzene ring's reactivity after the first alkyl group is added?
It becomes less reactive
It remains the same
It becomes more reactive
It becomes deactivated
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is isopropyl benzene formed as the major product when benzene reacts with propyl chloride?
Because propyl chloride is more reactive
Because of a hydride shift leading to a more stable secondary carbocation
Due to the presence of excess aluminum chloride
Due to the formation of a primary carbocation
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a limitation of the Friedel-Crafts alkylation reaction?
Polyalkylation can occur
Carbocation rearrangements can occur
It works well with strongly deactivating groups
It can lead to a mixture of products
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
DNA Repair Mechanisms and Functions
Interactive video
•
10th - 12th Grade

11 questions
IUPAC Nomenclature of Alkenes and Alkynes
Interactive video
•
10th - 12th Grade

8 questions
Aromatics and Cyclic Compounds - Crash Course Chemistry
Interactive video
•
11th Grade - University

6 questions
Engineers investigate possible lingering impacts from Elk River chemical spill - Science Nation
Interactive video
•
11th Grade - University

6 questions
The Science Of REAL Hoverboards
Interactive video
•
11th Grade - University

6 questions
Understanding Benzene and Its Effects
Interactive video
•
9th - 12th Grade

11 questions
Determining SN1, SN2, E1, and E2 Reactions: Crash Course Organic Chemistry
Interactive video
•
11th Grade - University

10 questions
Friedel Crafts Reactions and Directing Effects
Interactive video
•
11th - 12th Grade
Popular Resources on Wayground

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

15 questions
Solving Equations with Variables on Both Sides Review
Quiz
•
8th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Unit 6-Review The Mole
Quiz
•
11th - 12th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

148 questions
Fall TEKS Review Chemistry
Quiz
•
9th - 12th Grade

20 questions
Unit 5 - Chemical Reactions Refresh
Quiz
•
9th - 12th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade