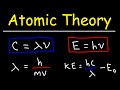
Mastering Atomic Theory: Key Formulas in Light and Energy
Interactive Video
•
Physics, Chemistry, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula to calculate the speed of light using wavelength and frequency?
C = Lambda + new
C = Lambda * new
C = Lambda / new
C = new / Lambda
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the energy of a photon calculated using frequency?
E = frequency / H
E = H * frequency
E = H / frequency
E = H + frequency
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the conversion factor from nanometers to meters?
1 nanometer = 1 * 10^-12 meters
1 nanometer = 1 * 10^-3 meters
1 nanometer = 1 * 10^-6 meters
1 nanometer = 1 * 10^-9 meters
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the photoelectric effect, what is the kinetic energy of an ejected electron equal to?
Work function minus energy of incoming photon
Energy of incoming photon minus work function
Energy of incoming photon plus work function
Energy of incoming photon times work function
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula for calculating the maximum wavelength needed to free an electron from a metal?
Max wavelength = speed of light / Plank's constant
Max wavelength = Plank's constant / speed of light
Max wavelength = Plank's constant * speed of light / threshold energy
Max wavelength = threshold energy / Plank's constant
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert work function from electron volts to joules?
Divide by 1.62 * 10^-19
Multiply by 1.62 * 10^-19
Divide by 1.62 * 10^19
Multiply by 1.62 * 10^19
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is De Broglie's wavelength formula?
Wavelength = speed / (Plank's constant * mass)
Wavelength = mass / (Plank's constant * speed)
Wavelength = Plank's constant / (mass * speed)
Wavelength = Plank's constant * mass * speed
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Light and Photons Concepts
Interactive video
•
11th - 12th Grade

11 questions
Photon Energy and Planck Constant Quiz
Interactive video
•
9th - 10th Grade

11 questions
Photon Momentum and Effective Mass
Interactive video
•
9th - 12th Grade

11 questions
Kinetic Energy and Photon Concepts
Interactive video
•
9th - 12th Grade

11 questions
Energy, Wavelength, and Constants in Physics
Interactive video
•
9th - 12th Grade

11 questions
Quantum Mechanics and Light
Interactive video
•
9th - 12th Grade

11 questions
The Bohr Model and Electron Transitions in Atomic Structure
Interactive video
•
9th - 12th Grade

11 questions
Understanding Laser Diodes
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

10 questions
Using Scalar and Vector Quantities
Quiz
•
8th - 12th Grade

14 questions
Distance & Displacement
Quiz
•
11th Grade

20 questions
Acceleration
Quiz
•
9th Grade

5 questions
Reading Motion Graphs
Lesson
•
8th - 10th Grade

8 questions
Distance Time Graphs
Lesson
•
9th - 12th Grade