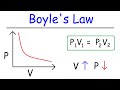
Boyle's Law and Its Impact on Gas Pressure and Volume Dynamics
Interactive Video
•
Physics, Chemistry, Science
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the pressure of a gas when the volume of its container is decreased, assuming temperature remains constant?
The pressure increases.
The pressure remains the same.
The pressure fluctuates randomly.
The pressure decreases.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following best describes Boyle's Law?
Pressure and volume are directly proportional.
Pressure and volume are inversely proportional.
Pressure and temperature are directly proportional.
Volume and temperature are inversely proportional.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the shape of the graph that represents Boyle's Law when plotting pressure against volume?
A straight line
A curved line
A zigzag line
A horizontal line
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the first problem-solving example, what was the initial pressure of the gas in the container?
1.6 atm
2.5 atm
4.6 atm
7.19 atm
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the first example, what was the new pressure after the volume was decreased to 1.6 liters?
7.19 atm
1.0 atm
2.5 atm
4.6 atm
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the second problem-solving example, what was the initial volume of the container?
1.6 liters
3.5 liters
4.83 liters
2.5 liters
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What was the final volume of the container in the second example after the pressure was decreased to 625 torr?
3.5 liters
1.6 liters
4.83 liters
2.5 liters
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Boyle's Law and Gas Properties
Interactive video
•
8th - 10th Grade

11 questions
Mastering The Ideal Gas Law Through Key Principles And Applications
Interactive video
•
9th - 10th Grade

11 questions
Gas Laws and Stoichiometry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Mastering Ideal Gas Laws Through Engaging Scenarios
Interactive video
•
9th - 10th Grade

9 questions
Mastering Boyle's Law Through Real-World Applications
Interactive video
•
9th - 10th Grade

11 questions
Understanding Gases and Gas Laws
Interactive video
•
9th - 10th Grade

6 questions
Boyle's Law and Gas Behavior
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Physics

15 questions
Metric Conversions
Quiz
•
9th Grade

10 questions
Significant Figures
Quiz
•
10th - 12th Grade

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

20 questions
Kinetic and Potential Energy
Quiz
•
9th - 12th Grade

15 questions
Distance and Displacement
Quiz
•
9th Grade

10 questions
Constant Velocity Motion
Quiz
•
9th - 11th Grade

15 questions
Warm Up Review Motion Graphs, Velocity, Speed
Quiz
•
9th - 12th Grade

29 questions
Phases of Matter
Quiz
•
8th - 10th Grade