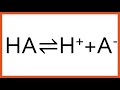
The Role and Function of Buffer Solutions in Chemical Reactions
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the color of a universal indicator when an acid is added to a neutral solution?
It turns blue
It turns red
It turns yellow
It remains green
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary function of a buffer solution?
To increase the pH of a solution
To decrease the pH of a solution
To change the color of a solution
To maintain a stable pH despite the addition of acids or alkalis
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does HA represent in a buffer solution?
A strong base
A weak acid
A strong acid
A neutral compound
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a buffer solution, what is the role of the weak acid HA when exposed to OH-?
It forms a strong acid
It remains unchanged
It forms water and a conjugate base
It forms a strong base
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens if an excessive amount of OH- is added to a buffer solution?
The buffer solution becomes more acidic
The buffer solution becomes more alkaline
The buffer solution remains unchanged
The buffer solution evaporates
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is a soluble salt added to a buffer solution?
To increase the acidity
To change the color of the solution
To decrease the alkalinity
To provide additional A- ions
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of the reaction between A- and H+ in a buffer solution?
No reaction occurs
Formation of a strong base
Formation of a weak acid
Formation of a strong acid
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Acids, Bases, and pH Concepts
Interactive video
•
9th - 10th Grade

11 questions
Microorganisms and pH Levels
Interactive video
•
9th - 10th Grade

8 questions
Acids, Bases, and pH Concepts
Interactive video
•
9th - 10th Grade

7 questions
Calcium Chloride Reactions and Properties
Interactive video
•
9th - 10th Grade

8 questions
Zinc Chloride and Acid-Base Reactions
Interactive video
•
9th - 10th Grade

8 questions
Neutralization Reactions and Silver Nitrate
Interactive video
•
9th - 10th Grade

8 questions
Calcium Perchlorate and pH Concepts
Interactive video
•
9th - 10th Grade

6 questions
Understanding pH and Acids
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade