
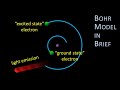
Bohr's Atomic Model and the Electromagnetic Spectrum Explained
Interactive Video
•
Physics, Chemistry, Science
•
9th - 10th Grade
•
Practice Problem
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the range of wavelengths in the electromagnetic spectrum?
From 1 kilometer to 1 millimeter
From 1 nanometer to 1 meter
From 100 meters to less than a trillionth of a meter
From 1 meter to 100 meters
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What did Bohr know about elements and light?
Electrons do not have energy
Energized elements emit unique colors of light
Light has no energy
Elements emit the same color of light
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the emission spectrum of hydrogen?
Only two colors: blue and green
Only one color: red
Four specific colors: violet, blue, blue-green, and red
A continuous rainbow of colors
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In Bohr's model, what happens when an electron transitions from a higher to a lower energy level?
The electron absorbs light
The electron emits light
The electron remains in the same orbit
The electron loses energy without emitting light
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What determines the color of light emitted during an electron transition?
The speed of the electron
The energy change of the electron
The size of the electron
The distance from the nucleus
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do electrons exist at discrete energy levels?
Because they are affected by gravity
Because they can only exist at specific allowed energies
Because they are constantly moving
Because they can exist at any energy level
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of integer-numbered orbits in Bohr's model?
They determine the electron's size
They have mathematical significance in representing electron energy
They represent the electron's speed
They have no significance
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Physics

10 questions
Exit Check 3.3 - Universal Gravitation
Quiz
•
9th Grade

10 questions
Exit Check 3.4 - Moon's Orbit
Quiz
•
9th Grade

10 questions
Exit Check 3.5 - Earth's Orbit
Quiz
•
9th Grade

22 questions
Waves
Quiz
•
KG - University

21 questions
EM Spectrum
Quiz
•
6th - 9th Grade

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

10 questions
Exploring the Properties of Waves
Interactive video
•
9th - 12th Grade

14 questions
Bill Nye Waves
Interactive video
•
9th - 12th Grade