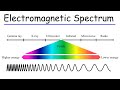
Electromagnetic Spectrum Dynamics and Photon Energy Relationships
Interactive Video
•
Physics, Science, Mathematics
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the speed of light in a vacuum?
3 x 10^8 meters per second
3 x 10^6 meters per second
3 x 10^4 meters per second
3 x 10^10 meters per second
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which type of electromagnetic radiation has the longest wavelength?
Microwaves
X-rays
Visible light
UV radiation
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
As you move towards gamma rays in the electromagnetic spectrum, what happens to the frequency?
It fluctuates
It remains the same
It decreases
It increases
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the relationship between the energy of a photon and its frequency?
Energy is directly proportional to frequency
Energy is equal to frequency
Energy is inversely proportional to frequency
Energy is unrelated to frequency
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following has the highest frequency?
Infrared radiation
Radio waves
UV radiation
X-rays
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Among the colors of visible light, which one has the highest energy?
Green
Red
Blue
Yellow
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the frequency of a photon given its wavelength?
Subtract the speed of light from the wavelength
Multiply the speed of light by the wavelength
Add the speed of light to the wavelength
Divide the speed of light by the wavelength
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Photoelectric Effect and Light Wavelengths
Interactive video
•
10th - 12th Grade

11 questions
Wave Particle Duality of Light: Exploring Its Properties and Phenomena
Interactive video
•
9th - 12th Grade

11 questions
Quantum Mechanics and Electron Configuration
Interactive video
•
9th - 12th Grade

11 questions
Ionization of Hydrogen Atom Quiz
Interactive video
•
10th - 12th Grade

11 questions
Mastering Wave Properties and Their Applications
Interactive video
•
9th - 12th Grade

11 questions
Photoelectric Effect Concepts
Interactive video
•
11th - 12th Grade

11 questions
Exploring The Electromagnetic Spectrum: Energy, Wavelength, And Frequency
Interactive video
•
9th - 12th Grade

11 questions
Laser Light and Atomic Reactions
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

10 questions
Using Scalar and Vector Quantities
Quiz
•
8th - 12th Grade

14 questions
Distance & Displacement
Quiz
•
11th Grade

20 questions
Acceleration
Quiz
•
9th Grade

5 questions
Reading Motion Graphs
Lesson
•
8th - 10th Grade

8 questions
Distance Time Graphs
Lesson
•
9th - 12th Grade