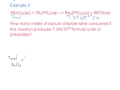
Stoichiometry Concepts and Calculations
Interactive Video
•
Chemistry, Science, Mathematics
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of lesson 9.3 in stoichiometry?
Balancing chemical equations
Calculating molar mass
Understanding mixed inputs and outputs
Identifying reaction types
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a typical output in stoichiometry problems?
Volume
Number of particles
Color change
Mass
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which tool is essential for solving stoichiometry problems?
A ruler
A thermometer
A microscope
A periodic table
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of using a mole map in stoichiometry problems?
To balance equations
To measure temperature
To identify reaction types
To convert between different units
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the calcium carbonate reaction example, what is the first step after writing the chemical equation?
Measuring the temperature
Balancing the equation
Calculating the molar mass
Identifying the precipitate
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many liters of CO2 are produced from 12.3 grams of calcium carbonate at STP?
1.5 liters
3.0 liters
4.0 liters
2.75 liters
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the barium chloride reaction, what is the precipitate formed?
Barium sulfate
Calcium chloride
Sodium chloride
Barium phosphate
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Moles and Molar Mass Concepts
Interactive video
•
9th - 12th Grade

11 questions
Understanding Mole Ratios in Stoichiometry
Interactive video
•
9th - 12th Grade

11 questions
Exploring Molarity and Dilution Concepts
Interactive video
•
9th - 12th Grade

11 questions
Volatilization and Precipitation Analysis
Interactive video
•
9th - 12th Grade

8 questions
Chemical Reactions and Stoichiometry
Interactive video
•
10th - 12th Grade

11 questions
Stoichiometry Concepts and Calculations
Interactive video
•
9th - 12th Grade

11 questions
Gravimetric Analysis and Stoichiometry
Interactive video
•
10th - 12th Grade

11 questions
Converting Grams to Moles with Stoichiometry
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade