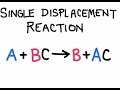
Reactivity and Compounds of Magnesium
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of the video tutorial?
Single displacement reactions
Double displacement reactions
Synthesis reactions
Combustion reactions
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a single displacement reaction, what typically happens to the lone element?
It decomposes into simpler substances
It forms a new compound with a non-metal
It replaces an element in a compound
It remains unchanged
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the activity series in predicting reactions?
It lists elements in alphabetical order
It shows the reactivity of metals
It determines the temperature of reactions
It predicts the color change in reactions
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is magnesium able to replace aluminum in a reaction?
Magnesium is less reactive than aluminum
Magnesium is more reactive than aluminum
Magnesium is heavier than aluminum
Magnesium is a non-metal
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is formed when magnesium reacts with chloride?
Metallic compound
Organic compound
Ionic compound
Covalent compound
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge does magnesium typically have in ionic compounds?
2-
1+
2+
1-
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a diatomic element?
Helium
Carbon
Sulfur
Iodine
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Activity Series and Metal Reactivity
Interactive video
•
9th - 10th Grade

11 questions
Types of Chemical Reactions
Interactive video
•
9th - 10th Grade

9 questions
Chemical Reactions of Sodium and Chlorine
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions and Diatomic Gases
Interactive video
•
9th - 10th Grade

11 questions
Understanding Chemical Compounds and Reactions
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

10 questions
Types of Chemical Reactions and Oxidation States
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions Involving Magnesium
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade