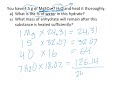
Understanding Hydrates and Water Composition
Interactive Video
•
Chemistry, Science, Mathematics
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are hydrates typically composed of?
Organic compounds with carbon
Covalent compounds with no water
Metallic compounds with oxygen
Ionic compounds with water molecules
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the water molecules in magnesium sulfate heptahydrate when heated?
They dissolve in the compound
They evaporate or boil away
They become more strongly bonded
They form a solid
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many water molecules are attached to each MgSO4 in magnesium sulfate heptahydrate?
Six
Seven
Eight
Five
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of water used in the calculation?
18.02 g/mol
20.00 g/mol
22.04 g/mol
16.00 g/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total mass of the hydrate including water?
200.00 g
246.52 g
150.00 g
300.00 g
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What percentage of the hydrate's mass is water?
45.00%
70.00%
51.17%
60.00%
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If you have 4.5 grams of the hydrate, how much of it is water?
4.0 grams
3.0 grams
2.3 grams
1.5 grams
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Chemical Nomenclature and Oxyanions
Interactive video
•
9th - 12th Grade

11 questions
Understanding Hydrates and Their Properties
Interactive video
•
9th - 10th Grade

8 questions
Plaster of Paris, Deliquescence and Efflorescence
Interactive video
•
9th - 10th Grade

8 questions
Carbohydrates Part 1: Simple Sugars and Fischer Projections
Interactive video
•
11th Grade - University

11 questions
Ocean Processes and Research Insights
Interactive video
•
9th - 10th Grade

11 questions
Percent Composition and Hydrates
Interactive video
•
9th - 10th Grade

10 questions
Molar Mass and Chemical Formulas
Interactive video
•
9th - 10th Grade

8 questions
Percent Composition of Magnesium Sulfate Heptahydrate
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade