Electrolytes and Ionization Concepts
Interactive Video
•
Chemistry, Science, Biology
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary role of free ions in an electrolyte?
To form solid compounds
To conduct electricity
To evaporate quickly
To dissolve in water
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to glucose when it is dissolved in water?
It forms ions
It remains as glucose surrounded by water molecules
It evaporates
It turns into a gas
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of electrolyte is formed when sodium chloride is dissolved in water?
Non-electrolyte
Weak electrolyte
No electrolyte
Strong electrolyte
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a single reaction arrow indicate in a chemical equation?
Reversible reaction
Partial conversion of reactants to products
Complete conversion of reactants to products
No reaction occurs
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
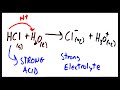
What happens when HCl is dissolved in water?
It forms a strong electrolyte
It forms a weak electrolyte
It does not dissolve
It forms a non-electrolyte
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of molecular ionization with a strong acid?
Formation of a non-electrolyte
Formation of a strong electrolyte
No ion formation
Formation of a weak electrolyte
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What distinguishes a weak acid from a strong acid in terms of ion formation?
Weak acids form ions rapidly
Weak acids form ions partially
Weak acids do not form ions
Weak acids form ions completely
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Electrolytes and Non-Electrolytes Concepts
Interactive video
•
9th - 10th Grade

8 questions
Properties and Behavior of NaBr in Water
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions and Ionic Equations
Interactive video
•
10th - 12th Grade

11 questions
Identifying Electrolytes: Strong, Weak, and Non-Electrolyte Characteristics
Interactive video
•
9th - 12th Grade

11 questions
Electrolytes and Net Ionic Equations
Interactive video
•
10th - 12th Grade

6 questions
Electrolytic and Solubility Properties Quiz
Interactive video
•
9th - 10th Grade

9 questions
Solubility and Electrolyte Properties of PbI2
Interactive video
•
9th - 10th Grade

9 questions
Properties and Behavior of MgSO4
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade