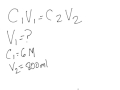
Dilution and Concentration Concepts
Interactive Video
•
Chemistry, Mathematics, Science
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary purpose of dilution in solution preparation?
To increase the concentration of a solution
To prepare a solution with a lower concentration
To change the color of a solution
To increase the volume of a solution without changing concentration
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the dilution formula C1V1 = C2V2, what does C1 represent?
The concentration of the diluted solution
The volume of the diluted solution
The volume of the stock solution
The concentration of the stock solution
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is typically true about the concentration of a stock solution?
It is the same as the diluted solution
It is lower than the diluted solution
It is higher than the diluted solution
It has no relation to the diluted solution
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the given problem, what is the concentration of the contact lens rinsing solution?
1 molar
800 molar
6 molar
0.15 molar
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the volume of the solution you wish to prepare in the problem?
200 ml
600 ml
800 ml
1000 ml
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the volume of the stock solution needed using the formula?
V1 = C1 * V2 / C2
V1 = C2 / C1 * V2
V1 = C1 / C2 * V2
V1 = C2 * V2 / C1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the calculated volume of the stock solution needed in the problem?
30 ml
20 ml
10 ml
40 ml
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Acids and Bases Concepts
Interactive video
•
9th - 10th Grade

6 questions
Duchess of Cornwall visits Tottenham Vaccination Centre
Interactive video
•
9th - 10th Grade

11 questions
Dilution and Concentration Calculations
Interactive video
•
9th - 10th Grade

11 questions
Understanding IBD and Dilution Concepts
Interactive video
•
9th - 10th Grade

11 questions
Preparing and Understanding Stock Solutions
Interactive video
•
9th - 10th Grade

11 questions
Bacterial Dilution Techniques and Calculations
Interactive video
•
9th - 10th Grade

11 questions
Chemical Reactions and Stoichiometry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Acids, Bases, and Reactions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade