Atmospheric Science Concepts Assessment
Interactive Video
•
Physics, Science, Chemistry
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary factor that causes the temperature to rise in the morning?
The incoming sunlight
The movement of tectonic plates
The rotation of the Earth
The gravitational pull of the moon
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
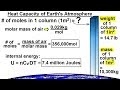
What does the heat capacity of the Earth's atmosphere determine?
The speed of wind
The amount of sunlight received
The temperature change with added heat
The color of the sky
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atmospheric pressure at sea level in Pascals?
1013 Pascals
1300 Pascals
101300 Pascals
13000 Pascals
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the mass of a column of air calculated?
By multiplying the weight by the acceleration due to gravity
By subtracting the weight from the acceleration due to gravity
By dividing the weight by the acceleration due to gravity
By adding the weight and the acceleration due to gravity
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the average molar mass of air?
8 grams per mole
32 grams per mole
29 grams per mole
18 grams per mole
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of air are in a column with a cross-sectional area of one square meter?
1,000,000 moles
100,000 moles
356,000 moles
500,000 moles
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the heat capacity at constant volume for diatomic molecules in the atmosphere?
3/2 times the universal gas constant
5/2 times the universal gas constant
7/2 times the universal gas constant
9/2 times the universal gas constant
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Exploring the Ideal Gas Law and Its Applications
Interactive video
•
9th - 12th Grade

11 questions
Understanding the Ideal Gas Law
Interactive video
•
9th - 12th Grade

11 questions
Gas Stoichiometry Problem Solving Techniques
Interactive video
•
9th - 12th Grade

11 questions
Avogadro's Law and Gas Behavior
Interactive video
•
9th - 12th Grade

6 questions
Understanding Gases and Pressure
Interactive video
•
9th - 10th Grade

11 questions
Understanding STP and Gas Laws
Interactive video
•
9th - 12th Grade

6 questions
Avogadro's Number
Interactive video
•
KG - University

11 questions
Molarity and Dilution Concepts
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

10 questions
Using Scalar and Vector Quantities
Quiz
•
8th - 12th Grade

14 questions
Distance & Displacement
Quiz
•
11th Grade

20 questions
Acceleration
Quiz
•
9th Grade

5 questions
Reading Motion Graphs
Lesson
•
8th - 10th Grade

8 questions
Distance Time Graphs
Lesson
•
9th - 12th Grade