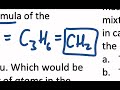
Mole Concepts and Empirical Formulas
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the relationship between the mass of a substance and its moles?
Mass is directly proportional to moles.
Mass is inversely proportional to moles.
Mass has no relation to moles.
Mass is equal to moles.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many particles are there in one mole of a substance?
6.02 x 10^25
6.02 x 10^23
6.02 x 10^22
6.02 x 10^24
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the percent composition of carbon in methane (CH4)?
25%
100%
75%
50%
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you determine the empirical formula from percent composition?
Use the periodic table to find the empirical formula.
Convert percentages to moles directly.
Convert percentages to grams, then to moles, and find the simplest ratio.
Empirical formulas cannot be determined from percent composition.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a balanced reaction, what does a mole ratio represent?
The ratio of densities of reactants and products.
The ratio of masses of reactants and products.
The ratio of moles of reactants and products.
The ratio of volumes of reactants and products.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the empirical formula of a compound with 36 grams of carbon and 6 grams of hydrogen?
C3H6
CH2
C2H4
C6H12
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is necessary to find the number of atoms in a sample of copper?
The volume of copper.
The molar mass of copper.
The density of copper.
The color of copper.
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Chemical Composition and Molar Mass
Interactive video
•
10th - 12th Grade

11 questions
Percent Composition and Molar Mass
Interactive video
•
9th - 12th Grade

8 questions
Chemistry - Calculating the Empirical Formula of a Compound!
Interactive video
•
10th Grade - University

11 questions
Empirical and Molecular Formulas
Interactive video
•
9th - 12th Grade

11 questions
Eugenol Chemistry Concepts
Interactive video
•
9th - 12th Grade

11 questions
Decoding Molecular Formulas Through Empirical Analysis
Interactive video
•
9th - 12th Grade

11 questions
Empirical and Molecular Formulas
Interactive video
•
9th - 12th Grade

11 questions
Empirical and Molecular Formulas
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade