Understanding Isotopes and Mass Spectrometry
Interactive Video
•
Chemistry, Physics, Science
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What did Dalton originally believe distinguished atoms of different elements?
Their color
Their charge
Their volume
Their mass
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Who is credited with the invention of mass spectrometry?
JJ Thompson
Niels Bohr
Ernest Rutherford
Albert Einstein
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In mass spectrometry, what happens to a particle that does not have a charge?
It continues to move straight and collides with the wall
It gets deflected by the magnetic field
It disappears
It changes its mass
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the degree of deflection in mass spectrometry depend on?
The temperature of the particle
The mass of the particle
The speed of the particle
The color of the particle
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
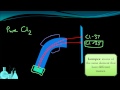
What did the mass spectrometry experiment with chlorine gas reveal?
Chlorine has only one isotope
Chlorine has two isotopes with different masses
Chlorine is not an element
Chlorine particles do not get ionized
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are isotopes?
Atoms with no neutrons
Atoms with no protons
Atoms of the same element with different masses
Atoms of different elements with the same mass
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many protons does a carbon atom always have?
Five
Six
Seven
Eight
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Chlorine Isotopes and Atomic Structure
Interactive video
•
9th - 10th Grade

11 questions
Chlorine Atom Mass and Isotopes
Interactive video
•
9th - 10th Grade

11 questions
Isotopes and Mass Spectrometry Concepts
Interactive video
•
10th - 12th Grade

11 questions
Relative Atomic and Isotopic Mass Concepts
Interactive video
•
9th - 10th Grade

11 questions
Understanding Atomic Structure and Isotopes
Interactive video
•
9th - 10th Grade

11 questions
Isotopes and Mass Spectrometry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Mass Spectrometry and Isotopes Concepts
Interactive video
•
9th - 10th Grade

11 questions
Mass Spectrometry and Isotopes Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade