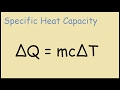
Heating Water and Energy Calculations
Interactive Video
•
Physics, Science, Mathematics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial temperature of the water in the hot water tank?
30°C
40°C
20°C
10°C
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which variable in the specific heat equation represents the mass of the substance?
C
Delta T
m
Delta Q
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the mass of the water calculated from its volume?
Multiply by 2
Divide by 2
Use the density of water
Use the specific heat capacity
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the specific heat capacity of water used in the calculation?
3,000 J/kg K
4,200 J/kg K
6,000 J/kg K
5,000 J/kg K
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the change in temperature (Delta T) for the water in this problem?
50°C
60°C
40°C
30°C
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much energy is required to raise the temperature of the water to 70°C?
18,350,000 J
28,350,000 J
38,350,000 J
48,350,000 J
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula for calculating power in terms of energy and time?
Power = Time / Energy
Power = Energy + Time
Power = Energy x Time
Power = Energy / Time
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Heat Transfer and Specific Heat
Interactive video
•
9th - 10th Grade

11 questions
Phase Changes and Heat Calculations
Interactive video
•
9th - 10th Grade

6 questions
GCSE Physics - Internal Energy and Specific Heat Capacity #28
Interactive video
•
9th - 10th Grade

11 questions
Determining Specific Heat Capacity Through Practical Experimentation
Interactive video
•
9th - 10th Grade

11 questions
Thermal Energy and Specific Heat
Interactive video
•
9th - 10th Grade

11 questions
Understanding Specific Heat Capacity Concepts
Interactive video
•
9th - 10th Grade

9 questions
Thermal Energy and Specific Heat
Interactive video
•
9th - 10th Grade

11 questions
Thermal Physics Concepts and Calculations
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade