Atomic Mass and Isotopes Concepts
Interactive Video
•
Chemistry, Physics, Mathematics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the standard element used to define atomic mass?
Nitrogen-14
Carbon-12
Oxygen-16
Hydrogen-1
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is AMU preferred over other units for atomic mass?
It is the only unit available.
It is easier to use and understand.
It is more accurate.
It is used only for carbon.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What distinguishes isotopes of the same element?
Different numbers of electrons
Different numbers of protons
Different numbers of neutrons
Different atomic numbers
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the average atomic mass on the periodic table determined?
By using a weighted average
By using the most common isotope
By using a simple average
By listing all isotopes
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a real-world example used to explain weighted averages?
Counting stars
Measuring temperature
GPA in school
Calculating speed
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
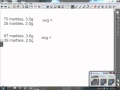
In the marble example, why is the average not simply the mean of the two weights?
Because the weights are not in grams
Because the marbles are not round
Because the marbles are different colors
Because the weights are not equal
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the key factor in calculating a weighted average for isotopes?
The abundance of each isotope
The color of the isotope
The charge of the isotope
The size of the isotope
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Understanding Atomic Structure and Isotopes
Interactive video
•
9th - 10th Grade

11 questions
Isotopic Abundance and Atomic Mass
Interactive video
•
9th - 10th Grade

11 questions
Silver Isotopes and Atomic Structure
Interactive video
•
9th - 10th Grade

11 questions
Atomic Structure and Isotopes
Interactive video
•
9th - 10th Grade

11 questions
Iron Isotopes and Atomic Structure
Interactive video
•
9th - 10th Grade

11 questions
Atomic Structure and Chemical Properties
Interactive video
•
9th - 10th Grade

6 questions
Understanding the Periodic Table
Interactive video
•
9th - 10th Grade

11 questions
Understanding Nitrogen: Atomic Structure
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade