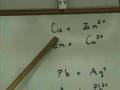
Reactivity and Spontaneity in Chemistry
Interactive Video
•
Chemistry, Science, Biology
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary purpose of Table J in chemistry?
To list elements alphabetically
To show the reactivity order of elements
To display atomic weights
To categorize elements by color
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why are group one metals considered highly reactive?
They have high electronegativities
They are located at the bottom of the periodic table
They have multiple valence electrons
They easily donate their single valence electron
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element is known for having the highest desire for electrons?
Chlorine
Oxygen
Fluorine
Nitrogen
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does a spontaneous reaction mean in practical terms?
It only occurs at high temperatures
It occurs without additional energy once started
It requires continuous energy input
It never occurs naturally
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is an example of a non-spontaneous process?
A candle burning
A battery being recharged
Ice melting at room temperature
A battery powering a device
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a spontaneous reaction, which element should be in the elemental state?
The element with the highest atomic number
The element higher in the chart
The element lower in the chart
The element with the lowest atomic weight
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does zinc react spontaneously with copper ions?
Zinc is less reactive than copper
Copper has more electrons to donate
Copper is higher in the reactivity series
Zinc is higher in the reactivity series
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade