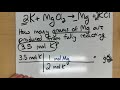
Stoichiometry Problem Solving Steps
Interactive Video
•
Chemistry, Science, Mathematics
•
9th - 10th Grade
•
Practice Problem
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in solving a stoichiometry problem after obtaining a balanced equation?
Calculate the mass of the desired substance
Convert to the requested units
Use a mole ratio
Convert the given substance to moles
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction of potassium and magnesium chloride, what is the mole ratio of potassium to magnesium?
3:1
1:2
2:1
1:1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When converting moles of magnesium to grams, which tool is used to find the molar mass?
Mole Ratio
Calculator
Periodic Table
Chemical Equation
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the decomposition of copper(II) oxide, what is the first step if you start with grams?
Convert moles to grams
Find the balanced equation
Use a mole ratio
Convert grams to moles
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of copper(II) oxide used in the example?
32.00 g/mol
63.55 g/mol
16.00 g/mol
79.55 g/mol
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the problem asks for grams of oxygen instead of moles, what additional step is needed?
Recalculate the mole ratio
Find a new balanced equation
Convert moles to grams
Use a different mole ratio
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of oxygen used when converting moles to grams?
64.00 g/mol
8.00 g/mol
32.00 g/mol
16.00 g/mol
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
Understanding Infinite Geometric Series
Interactive video
•
9th - 10th Grade

11 questions
Understanding the Exact Area of a Yellow Ring
Interactive video
•
9th - 10th Grade

11 questions
Understanding Avogadro's Number and the Mole
Interactive video
•
9th - 10th Grade

11 questions
Rocket Propellant Chemistry Quiz
Interactive video
•
9th - 10th Grade

11 questions
Understanding P Series and Related Concepts
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass and Calculations Quiz
Interactive video
•
9th - 10th Grade

6 questions
Understanding Observations in Science
Interactive video
•
9th - 10th Grade

6 questions
Understanding Solutions and Suspensions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Formative 3BC: Ionic v Covalent Bonds
Quiz
•
9th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Mixed Bonding Naming
Quiz
•
9th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Chemical Reactions
Quiz
•
9th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade