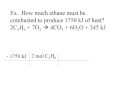
Chemical Reactions and Energy Changes
Interactive Video
•
Chemistry, Science, Physics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the two main types of chemical reactions based on energy changes?
Endothermic and Exothermic
Combustion and Synthesis
Decomposition and Displacement
Oxidation and Reduction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In which unit is energy most commonly measured in chemical reactions?
Kilojoules
Joules
Watts
Calories
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the energy of products compared to reactants in an endothermic reaction?
Energy is released
Energy remains the same
Products have more energy
Products have less energy
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the sign of Delta H in an endothermic reaction?
Positive
Undefined
Negative
Zero
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In an exothermic reaction, how does the energy of the products compare to the reactants?
Energy remains constant
Energy is absorbed
Products have less energy
Products have more energy
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the sign of Delta H in an exothermic reaction?
Negative
Zero
Positive
Undefined
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of ethane are needed to produce 1750 kilojoules of heat?
4.89 moles
2.54 moles
3.21 moles
5.07 moles
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
GCSE Chemistry - Reversible Reactions and Equilibrium #49
Interactive video
•
9th - 10th Grade

11 questions
Thermal Energy and Reactions Concepts
Interactive video
•
9th - 10th Grade

11 questions
Energy Transformations and Reactions
Interactive video
•
9th - 10th Grade

11 questions
Exothermic Reactions and Energy Diagrams
Interactive video
•
9th - 10th Grade

8 questions
GCSE Chemistry - Exothermic and Endothermic Reactions #43
Interactive video
•
9th - 10th Grade

9 questions
Candle Burning and Energy Changes
Interactive video
•
9th - 10th Grade

11 questions
Exothermic and Endothermic Reactions
Interactive video
•
9th - 10th Grade

11 questions
Endothermic and Exothermic Reactions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade