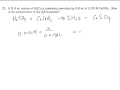
Titration Calculations and Concepts
Interactive Video
•
Chemistry, Mathematics, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in a titration calculation?
Measure the temperature of the solution
Determine the color change of the indicator
Write and balance the chemical equation
Calculate the pH of the solution
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a titration involving NaOH and HCl, what is the product formed besides water?
Sodium chloride
Sodium acetate
Sodium hydroxide
Hydrochloric acid
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert milliliters to liters in a titration calculation?
Multiply by 1000
Divide by 1000
Subtract 1000
Add 1000
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molarity of a solution if 0.01 moles of solute is dissolved in 0.038 liters of solution?
0.26 M
0.32 M
0.29 M
0.35 M
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the titration of NaOH with acetic acid, what is the salt formed?
Sodium sulfate
Sodium bicarbonate
Sodium chloride
Sodium acetate
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the stoichiometric ratio of acetic acid to sodium hydroxide in their titration?
2:1
1:2
1:1
3:1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the titration of sulfuric acid with calcium hydroxide, what is the balanced formula for the salt formed?
CaCl2
CaCO3
Ca(NO3)2
CaSO4
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Understanding Polyprotic Acids and Titration Curves
Interactive video
•
10th Grade - University

8 questions
Neutralization Reactions and Normality
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
10th - 12th Grade

11 questions
Acids and Bases Concepts
Interactive video
•
9th - 12th Grade

11 questions
Stoichiometry and pH Concepts
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Reactions and Ionic Equations
Interactive video
•
10th - 12th Grade

11 questions
Titration and pH Concepts
Interactive video
•
10th - 12th Grade

11 questions
Titration Concepts and Equivalence Points
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade