Thermochemistry and Calorimetry Concepts
Interactive Video
•
Chemistry, Physics, Science
•
10th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
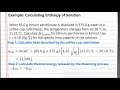
What is the initial temperature of the water in the calorimeter?
25.00 degrees Celsius
20.18 degrees Celsius
31.21 degrees Celsius
15.00 degrees Celsius
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of using a coffee-cup calorimeter in this experiment?
To find the density of lithium perchlorate
To determine the boiling point of water
To calculate the enthalpy of solution
To measure the mass of the solution
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total mass of the solution in the calorimeter?
85.0 grams
375.0 grams
500.0 grams
460.0 grams
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much heat is absorbed by the calorimeter's contents?
21,208 joules
15,000 joules
30,000 joules
10,000 joules
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the dissolving process considered exothermic?
Because it absorbs heat from the surroundings
Because it requires energy input to dissolve
Because it releases thermal energy, increasing the temperature
Because it decreases the temperature of the solution
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the sign of the energy released by the dissolving process?
Negative
Positive
Undefined
Zero
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the heat released per 85.0 grams of lithium perchlorate?
15,000 joules
21,208 joules
-15,000 joules
-21,208 joules
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert joules to kilojoules?
Divide by 1000
Multiply by 1000
Subtract 1000
Add 1000
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar enthalpy of solution for lithium perchlorate?
-26.5 kilojoules per mole
26.5 kilojoules per mole
-21.5 kilojoules per mole
21.5 kilojoules per mole
Similar Resources on Wayground

6 questions
Interactions of Electromagnetic Waves with Charged Particles
Interactive video
•
10th Grade - University

8 questions
Introduction to Work with Examples
Interactive video
•
11th Grade - University

8 questions
IIT/JEE Chemistry Practice #25: Enthalpy of Formation
Interactive video
•
11th Grade - University

5 questions
Discolouration
Interactive video
•
KG - 12th Grade

6 questions
I WONDER - Do All Penguins Live In Cold Places? Me Pregunto - Todos Los Pingüinos Viven En Lugares Fríos?
Interactive video
•
KG - 12th Grade

6 questions
I WONDER - When Did The Allosaurus Roam The Earth? Me Pregunto - Cuándo Vagó El Allosaurus Por La Tierra?
Interactive video
•
KG - 12th Grade

6 questions
I WONDER - What Job Does Blood Do? Me Pregunto - Qué Trabajo Hace La Sangre?
Interactive video
•
KG - 12th Grade

6 questions
Chemistry - The Mole Explained - What is Avogadro's Number?!
Interactive video
•
10th Grade - University
Popular Resources on Wayground

20 questions
Halloween Trivia
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Order of Operations
Quiz
•
5th Grade

20 questions
Halloween
Quiz
•
5th Grade

16 questions
Halloween
Quiz
•
3rd Grade

12 questions
It's The Great Pumpkin Charlie Brown
Quiz
•
1st - 5th Grade

20 questions
Possessive Nouns
Quiz
•
5th Grade

10 questions
Halloween Traditions and Origins
Interactive video
•
5th - 10th Grade
Discover more resources for Chemistry

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade

20 questions
2.6 Electron Configurations and Orbital Notations
Quiz
•
10th Grade

55 questions
Unit 4: A Conflict of Cans Summative Review
Quiz
•
11th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

15 questions
Electron Configurations and Orbital Notation
Quiz
•
11th Grade

10 questions
Chemistry Halloween Quiz
Quiz
•
12th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade