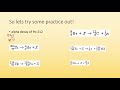
Nuclear Reactions and Transmutation Concepts
Interactive Video
•
Physics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary objective when dealing with transmutation equations?
To balance the number of atoms
To write and complete decay equations
To increase the atomic number
To change the color of elements
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In nuclear reactions, what must be balanced on both sides of the equation?
Temperature and pressure
Mass and charge
Volume and density
Speed and velocity
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When solving a decay equation, what is the first step in determining the unknown element X?
Balance the temperature
Check the periodic table
Balance the mass
Guess the element
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What famous equation explains the conversion of mass to energy in nuclear reactions?
F=ma
E=mc²
P=IV
V=IR
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is an example of a natural transmutation process?
Electrolysis
Alpha decay
Photosynthesis
Combustion
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic number of polonium used in the alpha decay example?
82
84
86
88
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the practice problem, what element is identified with an atomic number of 82?
Barium
Nitrogen
Lead
Radon
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Understanding Atomic Structure Concepts
Interactive video
•
9th - 10th Grade

11 questions
Balancing Nuclear Equations Through Alpha Decay
Interactive video
•
9th - 10th Grade

8 questions
GCSE Physics - Atomic Structure, Isotopes & Electrons Shells #32
Interactive video
•
9th - 10th Grade

11 questions
Nuclear Reactions and Decay Concepts
Interactive video
•
9th - 10th Grade

11 questions
Understanding Radioactivity and Radiation
Interactive video
•
9th - 10th Grade

8 questions
Atomic Masses and Composition of Nucleus
Interactive video
•
9th - 10th Grade

11 questions
Nuclear Decay Processes and Concepts
Interactive video
•
9th - 12th Grade

11 questions
Subatomic Particles and Their Properties
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

10 questions
Using Scalar and Vector Quantities
Quiz
•
8th - 12th Grade

20 questions
Acceleration
Quiz
•
9th Grade

5 questions
Reading Motion Graphs
Lesson
•
8th - 10th Grade

8 questions
Distance Time Graphs
Lesson
•
9th - 12th Grade

13 questions
Velocity Graphs Position vs. Time
Quiz
•
10th - 11th Grade