What is the first reactant always starting with in the Alex problem?
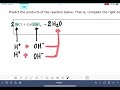
Ionic Reactions and Stoichiometry Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
A hydrogen ion
A calcium ion
A chloride ion
A hydroxide ion
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the second reactant always ending with in the Alex problem?
A chloride ion
A calcium ion
A hydroxide ion
A hydrogen ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the context of this problem, what do hydrogen and hydroxide ions combine to form?
Hydrogen peroxide
Calcium chloride
Water
Oxygen
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many water molecules are formed when two hydrogen ions combine with two hydroxide ions?
Four
Two
One
Three
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why might you need to adjust stoichiometric coefficients in this reaction?
To increase the reaction speed
To balance the equation
To change the reactants
To decrease the reaction temperature
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of calcium ions in the formation of the second product?
They dissolve in water
They are not involved
They form a gas
They combine with chloride ions
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the importance of stoichiometric coefficients in this reaction?
They ensure the reaction is balanced
They determine the color of the product
They decrease the pressure
They increase the temperature
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Balancing Chemical Equations with Polyatomic Ions
Interactive video
•
9th - 10th Grade

11 questions
Precipitation Reactions and Solubility
Interactive video
•
9th - 10th Grade

10 questions
Balancing Reactions with Sodium Hydroxide
Interactive video
•
9th - 10th Grade

9 questions
Balancing Chemical Reactions
Interactive video
•
9th - 10th Grade

9 questions
Chemical Reactions and Ionic Charges
Interactive video
•
9th - 10th Grade

11 questions
Acid-Base Reactions and Concepts
Interactive video
•
9th - 10th Grade

10 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

7 questions
Properties of Potassium Hydroxide
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

10 questions
Writing and Identifying Ratios Practice
Quiz
•
5th - 6th Grade

36 questions
Prime and Composite Numbers
Quiz
•
5th Grade

14 questions
Exterior and Interior angles of Polygons
Quiz
•
8th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade
Discover more resources for Chemistry

25 questions
Equations of Circles
Quiz
•
10th - 11th Grade

30 questions
Week 5 Memory Builder 1 (Multiplication and Division Facts)
Quiz
•
9th Grade

33 questions
Unit 3 Summative - Summer School: Immune System
Quiz
•
10th Grade

37 questions
Camp Re-cap Week 1 (no regression)
Quiz
•
9th - 12th Grade

46 questions
Biology Semester 1 Review
Quiz
•
10th Grade