Titration Concepts and Calculations
Interactive Video
•
Chemistry
•
11th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial concentration of acetic acid used in the titration experiment?
0.5 M
1.0 M
2.0 M
1.5 M
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What volume of NaOH is added to reach the equivalence point?
20 mL
25 mL
30 mL
35 mL
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
After the equivalence point, what type of problem is the titration considered?
Equilibrium problem
Stoichiometric problem
Kinetic problem
Thermodynamic problem
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
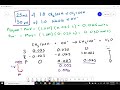
In the surf table, what is identified as the limiting reagent after the equivalence point?
Strong base (OH-)
Water
Weak acid
Acetate
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final concentration of OH- after the equivalence point?
0.1101 M
0.0909 M
0.0707 M
0.0505 M
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the pH calculated from the pOH in this titration?
pH = pOH + 14
pH = pOH - 7
pH = 14 - pOH
pH = 7 - pOH
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the pH of the solution after adding 30 mL of NaOH?
12.9
12.5
12.0
11.5
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Titration and Acid-Base Reactions
Interactive video
•
9th - 12th Grade

11 questions
Titration and Molar Ratios
Interactive video
•
10th - 12th Grade

11 questions
Transition Elements and Reaction Rates
Interactive video
•
11th Grade - University

11 questions
Mastering Acid-Base Equilibrium and Titration Concepts in Chemistry
Interactive video
•
9th - 12th Grade

8 questions
Titration of Strong Acid With Strong Base
Interactive video
•
11th Grade - University

11 questions
Understanding Acid-Base Titration
Interactive video
•
10th Grade - University

11 questions
Acid-Base Titration Concepts
Interactive video
•
10th - 12th Grade

11 questions
Buffer Systems and Their Functions
Interactive video
•
11th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Metric Conversions
Quiz
•
11th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

22 questions
SCIENCE LAB EQUIPMENT
Quiz
•
5th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

18 questions
Classifying Matter Particle Diagrams
Quiz
•
11th - 12th Grade

19 questions
U2 Protons Neutrons and Electrons
Quiz
•
11th Grade