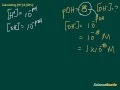
Understanding Ion Concentrations and Equations
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of this video tutorial?
Identifying chemical reactions
Balancing chemical equations
Understanding ion concentrations
Calculating molar mass
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which equation is used to find the hydrogen ion concentration?
Hydrogen ion concentration = 10^pH
Hydrogen ion concentration = 10^-pH
Hydroxide ion concentration = 10^-pOH
Hydroxide ion concentration = 10^pOH
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the pH is 2, what is the hydrogen ion concentration?
1 * 10^-1 M
1 * 10^-2 M
1 * 10^-4 M
1 * 10^-3 M
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is a pH of 4 expressed in terms of hydrogen ion concentration?
0.1 M
0.0001 M
0.001 M
0.01 M
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which equation is used to find the hydroxide ion concentration?
Hydroxide ion concentration = 10^-pOH
Hydroxide ion concentration = 10^pH
Hydroxide ion concentration = 10^-pH
Hydroxide ion concentration = 10^pOH
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
For a pOH of 3, what is the hydroxide ion concentration?
1 * 10^-3 M
1 * 10^2 M
1 * 10^3 M
1 * 10^-2 M
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the hydroxide ion concentration for a pOH of 8?
1 * 10^-6 M
1 * 10^-5 M
1 * 10^-7 M
1 * 10^-8 M
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Acid-Base Theories and Concepts
Interactive video
•
9th - 10th Grade

11 questions
Acids and Bases Quiz
Interactive video
•
9th - 10th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Acids and Bases Concepts
Interactive video
•
9th - 10th Grade

11 questions
Calculating pOH and pH from Hydroxide Ion Concentration
Interactive video
•
9th - 10th Grade

11 questions
Hydronium Ion Concentration and pH
Interactive video
•
9th - 10th Grade

11 questions
Calculating pH in Acid-Base Reactions
Interactive video
•
10th - 11th Grade

10 questions
pH Levels and Hydrogen Ion Concentration in Aquatic Environments
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade