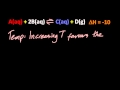
Le Chatelier's Principle and Equilibrium
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary purpose of Le Chatelier's Principle in equilibrium systems?
To calculate the exact concentration of reactants
To understand how systems respond to stress
To determine the speed of reactions
To predict the color change in reactions
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does increasing the concentration of a reactant affect the equilibrium position?
It has no effect on the equilibrium
It shifts the equilibrium to the right
It stops the reaction completely
It shifts the equilibrium to the left
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does removing a reactant affect the equilibrium position?
It shifts the equilibrium to the left
It shifts the equilibrium to the right
It has no effect on the equilibrium
It increases the reaction rate
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the equilibrium when the pressure is increased in a system with gases?
The equilibrium shifts to the side with more gas particles
The reaction stops
The equilibrium shifts to the side with fewer gas particles
The equilibrium remains unchanged
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of increasing pressure in a reaction with equal moles of gas on both sides?
The equilibrium shifts to the left
The reaction stops
The equilibrium shifts to the right
The equilibrium remains unchanged
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If a system at equilibrium is heated, which side of the reaction is favored?
The side that releases heat (exothermic)
The side with more products
The side that absorbs heat (endothermic)
The side with more reactants
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a reaction where the forward reaction is exothermic, what happens when the temperature is increased?
The equilibrium remains unchanged
The reaction stops
The equilibrium shifts to the left
The equilibrium shifts to the right
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Solubility and Common Ion Effects
Interactive video
•
10th - 12th Grade

6 questions
Equilibrium and Temperature Effects on Nitrogen Dioxide and Dinitrogen Tetroxide
Interactive video
•
9th - 12th Grade

11 questions
Equilibrium Constant Concepts
Interactive video
•
10th - 12th Grade

6 questions
Predicting direction of reaction
Interactive video
•
10th Grade - University

6 questions
Reaction Quotient - Predicting Reaction
Interactive video
•
10th Grade - University

11 questions
Le Chatelier's Principle and Equilibrium
Interactive video
•
10th - 12th Grade

11 questions
Understanding ICE Tables and Equilibrium Calculations
Interactive video
•
10th - 12th Grade

11 questions
Le Châtelier’s Principle and Chemical Equilibrium
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade