Ethanol Solution Preparation Concepts
Interactive Video
•
Biology
•
9th - 10th Grade
•
Practice Problem
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
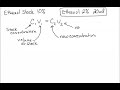
What is the primary purpose of using the C1V1 = C2V2 equation in the experiment described?
To find the volume of stock solution needed to create a new solution
To calculate the heart rate of daphnia
To measure the temperature of the ethanol solution
To determine the concentration of ethanol in the stock solution
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the 10% ethanol stock solution considered unsuitable for direct use with daphnia?
It is too expensive
It has too high of an ethanol concentration
It is not pure enough
It is too diluted
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the desired concentration of the new ethanol solution for the experiment?
5%
2%
10%
20%
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which variable represents the volume of the new solution to be made?
V1
C1
V2
C2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the known concentration of the stock solution in the problem?
2%
20%
10%
5%
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you simplify the equation to solve for V1?
Multiply all terms by 10
Cancel the percent signs and divide
Subtract the volumes
Add the concentrations
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the calculated volume of the stock solution needed to make the final solution?
8 milliliters
2 milliliters
4 milliliters
6 milliliters
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Biology

22 questions
Human Body Systems Overview
Quiz
•
9th Grade

25 questions
photosynthesis and cellular respiration
Quiz
•
9th Grade

15 questions
African American Impact in the 1980s
Quiz
•
9th Grade

20 questions
Symbiotic Relationships
Quiz
•
9th Grade

20 questions
Food Chains and Food Webs
Quiz
•
7th - 12th Grade

10 questions
Exploring Food Webs and Energy Pyramids
Interactive video
•
6th - 10th Grade

20 questions
Cladogram Practice
Quiz
•
10th Grade

20 questions
CFA #2 Unit 3 Human Body Systems (21.2 & 21.3)
Quiz
•
9th - 12th Grade