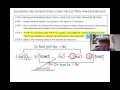
Balancing Redox Reactions and Oxidation States
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it often difficult to balance a redox reaction by observation alone?
Because the reactions are too fast to observe.
Because electron transfer is not easily observable.
Because the reactions are too slow to observe.
Because the oxidation numbers are not visible.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation state of sodium in a compound?
+1
+2
-1
0
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you determine the oxidation state of chlorine in NaClO4?
By looking at the periodic table.
By using the oxidation states of other elements in the compound.
By measuring the compound's pH.
By assuming it is always -1.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the oxidation number of a substance that is reduced?
It becomes zero.
It remains the same.
It decreases.
It increases.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the least common multiple used for in balancing redox reactions?
To determine the oxidation states.
To calculate the reaction rate.
To find the smallest number of atoms.
To equalize the number of electrons lost and gained.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the electron transfer method, what is the purpose of assigning coefficients?
To balance the number of atoms.
To balance the charges.
To balance the number of electrons.
To balance the reaction speed.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons does chlorine gain in the reduction process?
1
2
3
4
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Redox Reactions and Oxidation Numbers
Interactive video
•
9th - 12th Grade

6 questions
Practice Problem: Balancing Redox Reactions
Interactive video
•
11th Grade - University

11 questions
Redox Reactions and Their Characteristics
Interactive video
•
9th - 12th Grade

8 questions
Chemical Weathering Processes
Interactive video
•
11th Grade - University

11 questions
Balancing Redox Reactions Concepts
Interactive video
•
10th - 12th Grade

11 questions
Understanding Redox Reactions
Interactive video
•
9th - 12th Grade

11 questions
Oxidation States and Redox Reactions
Interactive video
•
10th - 12th Grade

11 questions
Understanding Galvanic Cells
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade