Effects of Pressure and Temperature on Water
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it easier to boil water on a mountain compared to sea level?
Water is purer on mountains.
There is less air pressure on mountains.
Mountains have more sunlight.
The temperature is higher on mountains.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the boiling point of water when air pressure is reduced?
It decreases.
It remains the same.
It increases.
It fluctuates randomly.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
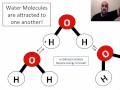
What is the charge distribution in a water molecule?
Both ends are positively charged.
Both ends are negatively charged.
There is no charge distribution.
One end is positive, and the other is negative.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary reason water molecules stick together?
Electrostatic force
Nuclear force
Gravitational pull
Magnetic attraction
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does surface tension affect the boiling point of water?
It makes water boil at room temperature.
It has no effect on the boiling point.
It decreases the boiling point.
It increases the boiling point.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What must be overcome for water to boil besides air pressure?
Magnetic fields
Surface tension
Electric currents
Gravity
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the effect of adding soap to water on its surface tension?
No effect on surface tension
Makes water solidify
Decreases surface tension
Increases surface tension
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Heating Curves and Energy Changes Quiz
Interactive video
•
9th - 10th Grade

11 questions
Phase Changes and Latent Heat Concepts
Interactive video
•
9th - 10th Grade

9 questions
Boiling Point and Pressure Concepts
Interactive video
•
9th - 10th Grade

7 questions
Boiling Water Under Reduced Pressure
Interactive video
•
9th - 10th Grade

11 questions
AP Chemistry Unit 3: Mixtures and Solubility
Interactive video
•
9th - 10th Grade

11 questions
Phase Changes and States of Matter
Interactive video
•
9th - 10th Grade

11 questions
Heating Curve Concepts and Phases
Interactive video
•
9th - 10th Grade

11 questions
Phases of Matter and Changes
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade