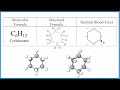
Cyclohexane Structure and Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Medium
Mia Campbell
Used 1+ times
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular formula of cyclohexane?
C5H10
C7H14
C6H12
C6H6
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the prefix 'cyclo' indicate in the name cyclohexane?
A double bond
A branched chain
A linear chain
A ring structure
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many carbon atoms are present in cyclohexane?
4
5
6
7
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bonds does each carbon atom form in cyclohexane?
2
3
5
4
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen atoms are bonded to each carbon atom in cyclohexane?
1
2
3
4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is not shown in the skeletal formula of cyclohexane?
Oxygen atoms
Both carbon and hydrogen atoms
Hydrogen atoms
Carbon atoms
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is another name for the skeletal formula?
Expanded formula
Bondline formula
Empirical formula
Molecular formula
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Gas Laws and Molar Concepts
Interactive video
•
9th - 10th Grade

11 questions
Density and Relative Density Quiz
Interactive video
•
9th - 10th Grade

6 questions
Human Body /Human Body Systems/Human Anatomy
Interactive video
•
KG - 9th Grade

2 questions
Phythaforas' Theorem: Finding the Circumference of a Circle
Interactive video
•
9th - 10th Grade

11 questions
The Beginning of Everything -- The Big Bang
Interactive video
•
9th Grade

11 questions
Understanding Distance Between Two Points
Interactive video
•
8th - 10th Grade

11 questions
Understanding Hess's Law and Enthalpy Changes
Interactive video
•
9th - 12th Grade

6 questions
Understanding Molecular and Empirical Formulas
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

10 questions
Formative 3BD: Ionic Bonds
Quiz
•
9th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade