Oxidation Numbers and Redox Reactions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
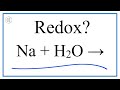
What is the main focus of the reaction between sodium and water discussed in the video?
Synthesis of a new compound
Acid-base reaction
Redox reaction analysis
Formation of a precipitate
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of a free element like sodium?
+2
0
-1
+1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When hydrogen is bonded to non-metals, what is its typical oxidation number?
-1
+2
0
+1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation number of oxygen in most compounds?
+1
-1
-2
0
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the oxidation number of sodium change in the reaction?
From -1 to 0
From +1 to 0
From 0 to -1
From 0 to +1
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the oxidation number of hydrogen in the reaction?
It increases from -1 to 0
It remains the same
It decreases from +1 to 0
It increases from 0 to +1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What indicates that a Redox reaction has occurred in this reaction?
No change in oxidation numbers
Formation of a gas
Change in oxidation numbers
Change in physical state
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of electron transfer in identifying a Redox reaction?
It suggests a change in color
It indicates a physical change
It confirms a Redox reaction
It shows a change in temperature
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What would indicate that the reaction is not a Redox reaction?
Production of light
No change in oxidation numbers
Formation of a solid
Change in temperature
Similar Resources on Wayground

11 questions
Redux Reactions and Oxidation Concepts
Interactive video
•
9th - 10th Grade

9 questions
Balancing Reactions of Magnesium and Nitrogen
Interactive video
•
9th - 10th Grade

10 questions
Oxidation Numbers and Redox Reactions
Interactive video
•
9th - 10th Grade

10 questions
Redox Reactions and Half-Reactions
Interactive video
•
9th - 10th Grade

6 questions
GCSE Chemistry - Oxidation and Reduction - Redox Reactions #39 (Higher Tier)
Interactive video
•
9th - 10th Grade

7 questions
Combustion and Exothermic Reactions
Interactive video
•
9th - 10th Grade

7 questions
Characteristics of Combustion Reactions
Interactive video
•
9th - 10th Grade

9 questions
Redox Reactions and Oxidation Numbers
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade