Lone Pairs and Molecular Geometry
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Emma Peterson
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
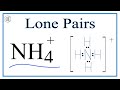
What is the charge of the ammonium ion (NH4+)?
Negative
Neutral
Variable
Positive
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of brackets in the Lewis structure of NH4+?
They indicate the presence of lone pairs.
They show the ion's charge.
They are used for aesthetic purposes.
They highlight the central atom.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs are present in the ammonium ion (NH4+)?
Three
None
Two
One
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which molecule contains a lone pair, NH4+ or NH3?
NH4+
NH3
Both
Neither
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What effect does the lone pair in NH3 have on its molecular geometry?
It creates a trigonal planar shape.
It has no effect on the shape.
It results in a trigonal pyramidal shape.
It makes the molecule linear.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In NH3, what role does the lone pair play in relation to the hydrogen atoms?
It has no interaction with them.
It repels them upwards.
It pushes them down.
It attracts them closer.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary difference in electron arrangement between NH4+ and NH3?
NH4+ has more lone pairs.
NH3 has a lone pair while NH4+ does not.
NH3 has fewer covalent bonds.
NH4+ has a different central atom.
Similar Resources on Wayground

9 questions
Ammonium Carbonate and Its Ions
Interactive video
•
9th - 10th Grade

10 questions
Molecular Geometry and Bonding Concepts
Interactive video
•
9th - 10th Grade

10 questions
Molecular Geometry of Propane
Interactive video
•
9th - 10th Grade

10 questions
Molar Mass and Chemical Formulas
Interactive video
•
9th - 10th Grade

8 questions
Experimental Design in Psychology
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry of N2H2
Interactive video
•
9th - 10th Grade

9 questions
Intermolecular Forces in Water and Ammonia
Interactive video
•
9th - 10th Grade

9 questions
Ionic Compounds and Bonding Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade