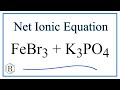
Net Ionic Equations and Spectator Ions
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing a net ionic equation?
Balance the molecular equation
Identify spectator ions
Determine the solubility of compounds
Write the complete ionic equation
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When balancing the molecular equation, what coefficient is placed in front of KBr?
1
2
4
3
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following compounds is generally insoluble unless paired with a group 1 element?
Bromide ion
Phosphate ion
Potassium phosphate
Iron(III) bromide
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the state of iron(III) phosphate in the reaction?
Solid
Aqueous
Gas
Liquid
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What do you do with strong electrolytes in the complete ionic equation?
Convert them to solids
Leave them as compounds
Split them into ions
Combine them
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ions are considered spectator ions in this reaction?
Iron(III) ions
Phosphate ions
Potassium ions
Bromide ions
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the net charge on both sides of the net ionic equation?
Variable
Zero
Negative
Positive
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
10th - 12th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
10th - 12th Grade

11 questions
Net Ionic Equations and Solubility
Interactive video
•
10th - 12th Grade

10 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
10th - 12th Grade

7 questions
Solubility and Net Ionic Equations
Interactive video
•
10th - 11th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
10th - 12th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
10th - 12th Grade

11 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade