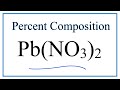
Percent Composition and Atomic Counts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Ethan Morris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in finding the percent composition of an element in a compound?
Add the atomic masses of all elements
Subtract the atomic number from the molar mass
Multiply the molar mass by the number of atoms
Divide the total mass by the number of atoms
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of lead (Pb) used in the calculation for Pb(NO3)2?
100.0 grams per mole
16.00 grams per mole
14.01 grams per mole
207.2 grams per mole
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lead atoms are present in Pb(NO3)2?
Four
Three
One
Two
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the percent composition of lead in Pb(NO3)2?
29.0%
8.46%
50.0%
62.5%
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many nitrogen atoms are there in Pb(NO3)2?
Four
Three
Two
One
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the percent composition of nitrogen in Pb(NO3)2?
62.5%
8.46%
29.0%
14.01%
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are present in Pb(NO3)2?
Eight
Six
Four
Two
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
Cambios físicos: recapitulando
Interactive video
•
10th - 12th Grade

8 questions
Composing Twelve-Tone Music - Including the Familiar
Interactive video
•
10th - 12th Grade

8 questions
The Difference Between 3:4 and 6:8 Time Signatures - Music Theory
Interactive video
•
10th - 12th Grade

8 questions
NIST Unscripted - Kent Irwin
Interactive video
•
10th - 12th Grade

6 questions
The Importance of Fresh Water
Interactive video
•
10th - 12th Grade

6 questions
CLEAN : Czech children get tested for Covid as kindergartens reopen
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Poles hold rally to defend sex education
Interactive video
•
9th - 10th Grade

6 questions
Properties of Gases and Air Composition
Interactive video
•
8th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Formative 3BC: Ionic v Covalent Bonds
Quiz
•
9th Grade

10 questions
Exploring Stoichiometry Concepts
Interactive video
•
6th - 10th Grade

20 questions
Mixed Bonding Naming
Quiz
•
9th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

20 questions
Chemical Reactions
Quiz
•
9th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Covalent Bonding
Quiz
•
10th Grade