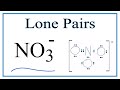
Molecular Geometry of Nitrate Ion
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the central atom in the nitrate ion?
Oxygen
Nitrogen
Carbon
Hydrogen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs are present on the central nitrogen atom in NO3-?
Two
Three
One
Zero
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs are found on the oxygen atom with a double bond in NO3-?
One
Two
Three
Four
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Do lone pairs on oxygen atoms significantly affect the molecular geometry of NO3-?
Depends on the temperature
Yes, they do
No, they don't
Only in certain conditions
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of the nitrate ion?
Bent
Tetrahedral
Trigonal planar
Linear
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle between atoms in the nitrate ion?
90 degrees
180 degrees
109.5 degrees
120 degrees
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If a lone pair were present on the central atom, what effect would it have on the molecular geometry?
It would push other atoms away
No effect
It would make the geometry tetrahedral
It would make the geometry linear
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

11 questions
Understanding Earth's Geometry and Circles
Interactive video
•
9th - 10th Grade

11 questions
Understanding the Standard Equation of a Circle
Interactive video
•
8th - 10th Grade

8 questions
Solving Quadratic Problems: Factored Form and Finding Zeros and Symmetry
Interactive video
•
9th - 10th Grade

9 questions
Behavior and Characteristics of Fish Eagles and Marabou Storks
Interactive video
•
9th - 10th Grade

11 questions
Balancing Equations in 5 Steps
Interactive video
•
10th Grade

11 questions
Pythagorean Theorem Concepts and Applications
Interactive video
•
9th - 10th Grade

11 questions
Understanding Ellipses and Their Properties
Interactive video
•
9th - 10th Grade

11 questions
Ellipse Properties and Translations
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

20 questions
MINERS Core Values Quiz
Quiz
•
8th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade

10 questions
How to Email your Teacher
Quiz
•
Professional Development

15 questions
Order of Operations
Quiz
•
5th Grade
Discover more resources for Chemistry

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

19 questions
Lewis Dot Structures -Review and Master
Quiz
•
10th Grade

10 questions
Electron Configuration, Orbital Notation, & Dot diagrams
Lesson
•
9th - 12th Grade

10 questions
Intro to Atoms Vocabulary Quiz
Quiz
•
8th - 10th Grade

20 questions
Naming Polyatomic Ionic compounds
Quiz
•
9th - 12th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade