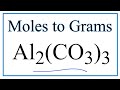
Moles and Grams Conversions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial quantity of aluminum carbonate given in moles for the conversion problem?
0.50 moles
0.63 moles
1.00 mole
2.00 moles
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of aluminum carbonate used in the conversion?
150.50 grams per mole
233.99 grams per mole
200.00 grams per mole
300.00 grams per mole
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert moles to grams in this context?
Subtract molar mass from moles
Divide moles by molar mass
Multiply moles by molar mass
Add moles to molar mass
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of converting 0.63 moles of aluminum carbonate to grams?
50.00 grams
200.00 grams
100.00 grams
147.41 grams
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If you have grams and need to find moles, what should you do?
Divide grams by molar mass
Multiply grams by molar mass
Subtract molar mass from grams
Add grams to molar mass
Similar Resources on Wayground

6 questions
Stoichiometry Conversion Quiz
Interactive video
•
9th - 10th Grade

8 questions
Molar Mass and Conversion Techniques
Interactive video
•
9th - 10th Grade

7 questions
Calculating Molar Mass of Propane
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass Calculations and Concepts
Interactive video
•
9th - 10th Grade

9 questions
Molar Mass and Conversion Concepts
Interactive video
•
9th - 10th Grade

6 questions
Understanding Argon and Molar Mass
Interactive video
•
9th - 10th Grade

7 questions
Molar Conversions and Molar Mass
Interactive video
•
9th - 10th Grade

6 questions
Empirical Formula Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade