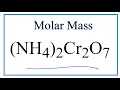
Molar Mass and Chemical Composition
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for ammonium dichromate?
NH4Cr2O7
(NH4)2Cr2O7
NH3Cr2O7
(NH3)2Cr2O7
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen atoms are present in ammonium dichromate?
2
4
6
8
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of nitrogen in ammonium dichromate?
14.01 g/mol
28.02 g/mol
18.05 g/mol
36.10 g/mol
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of chromium in ammonium dichromate?
32.00 g/mol
16.00 g/mol
103.98 g/mol
51.99 g/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are present in ammonium dichromate?
5
6
8
7
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total molar mass of ammonium dichromate?
150.00 g/mol
300.00 g/mol
200.00 g/mol
252.08 g/mol
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If you have 252.08 grams of ammonium dichromate, how many moles do you have?
3 moles
2 moles
0.5 moles
1 mole
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the molar mass in chemistry?
It shows the solubility of a compound.
It determines the color of a compound.
It helps in calculating the number of moles in a given mass.
It indicates the boiling point of a compound.
Similar Resources on Wayground

4 questions
Conservation of Matter Review Slides
Interactive video
•
8th Grade

6 questions
VOICED : Thousands flee Pakistan offensive
Interactive video
•
9th - 10th Grade

8 questions
After the fall of ISIS caliphate, its capital remains a city of the dead
Interactive video
•
9th - 10th Grade

2 questions
Sub-Atomic Particles – Protons and Neutrons
Interactive video
•
9th - 10th Grade

2 questions
CLEAN : Liberian women hold mass fast for peaceful elections
Interactive video
•
9th - 10th Grade

2 questions
Interview with mayor of London Sadiq Khan during his visit to mass vaccination centre at Chelsea’s football stadium
Interactive video
•
9th - 10th Grade

6 questions
Measuring in Science: Measuring Mass
Interactive video
•
10th - 12th Grade

6 questions
Introduction to Metric Mass and Volume
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade