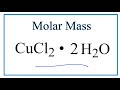
Molar Mass and Hydrates Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the classification of CuCl2.2H2O based on its water content?
Trihydrate
Dihydrate
Anhydrous
Monohydrate
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element's molar mass is 63.55 grams per mole?
Copper
Oxygen
Chlorine
Hydrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many chlorine atoms are present in CuCl2.2H2O?
One
Two
Four
Three
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of chlorine used in the calculation?
35.45 grams per mole
16.00 grams per mole
63.55 grams per mole
1.01 grams per mole
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of water used in the calculation?
9.01 grams per mole
36.04 grams per mole
72.08 grams per mole
18.02 grams per mole
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many water molecules are associated with each CuCl2 unit in CuCl2.2H2O?
Four
Three
Two
One
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total molar mass of CuCl2.2H2O as calculated in the video?
36.04 grams per mole
170.49 grams per mole
63.55 grams per mole
134.45 grams per mole
Create a free account and access millions of resources
Similar Resources on Wayground

6 questions
Molecular Weight of Calcium Chloride
Interactive video
•
9th - 10th Grade

8 questions
Percent Composition and Molar Mass
Interactive video
•
9th - 10th Grade

6 questions
Understanding Molar Mass of PbCl2
Interactive video
•
8th - 10th Grade

6 questions
Copper(II) Oxide Molar Mass and Properties
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Atomic Mass Concepts
Interactive video
•
9th - 10th Grade

6 questions
Zinc and Chlorine Molar Mass Calculations
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass of Chlorine and Cl2
Interactive video
•
9th - 10th Grade

9 questions
Molar Mass and Chemical Formulas
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade