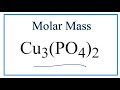
Copper II Phosphate Molar Mass Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in finding the molar mass of copper II phosphate?
Find the atomic masses of the elements from the periodic table.
Calculate the volume of the compound.
Determine the density of the compound.
Measure the temperature of the compound.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many copper atoms are present in copper II phosphate?
One
Two
Three
Four
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of copper used in the calculation?
60.00 grams per mole
63.55 grams per mole
65.38 grams per mole
58.44 grams per mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many phosphate ions are present in copper II phosphate?
Four
Three
Two
One
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of phosphorus used in the calculation?
31.00 grams per mole
32.07 grams per mole
33.00 grams per mole
30.97 grams per mole
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are present in one phosphate ion?
Two
Three
Four
Five
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total molar mass of copper II phosphate?
370.50 grams per mole
360.00 grams per mole
380.59 grams per mole
390.00 grams per mole
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of the phosphate ion in copper II phosphate?
189.94 grams per mole
180.00 grams per mole
200.00 grams per mole
170.00 grams per mole
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the final molar mass result?
It represents the mass of one mole of copper II phosphate.
It measures the temperature of copper II phosphate.
It indicates the density of copper II phosphate.
It shows the volume of copper II phosphate.
Similar Resources on Wayground

6 questions
Molar Mass and Compound Composition
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Atomic Contributions
Interactive video
•
9th - 10th Grade

7 questions
Understanding Urea: Molar Mass and Composition
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Chemical Composition
Interactive video
•
9th - 10th Grade

6 questions
Molar Mass and Atomic Structure
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Chemical Compounds
Interactive video
•
9th - 10th Grade

7 questions
Molar Mass and Atomic Structure
Interactive video
•
9th - 10th Grade

6 questions
Atomic Mass and Molar Calculations
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade