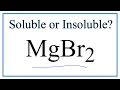
Solubility of MgBr2 in Water
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Aiden Montgomery
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main question addressed in the video regarding MgBr2?
Whether MgBr2 is soluble or insoluble in water.
Whether MgBr2 is reactive with air.
Whether MgBr2 is a solid or liquid.
Whether MgBr2 is a metal or non-metal.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which ion is generally soluble with few exceptions?
Nitrate ion
Bromide ion
Sulfate ion
Chloride ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following compounds is likely to be soluble in water based on the solubility rules discussed?
CaBr2
PbBr2
MgBr2
AgBr
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the solubility chart indicate about MgBr2?
It is soluble in water.
It forms a precipitate in water.
It is insoluble in water.
It reacts with water.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final conclusion about MgBr2's solubility in water?
MgBr2 is insoluble in water.
MgBr2 is partially soluble in water.
MgBr2 is soluble in water.
MgBr2 decomposes in water.
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade