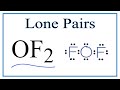
Lewis Structure and Molecular Geometry of OF2
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in analyzing the Lewis structure of OF2?
Counting the total number of electrons
Identifying the central atom
Starting with a valid Lewis structure
Determining the molecular geometry
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bonding pairs of electrons are present in OF2?
Four
Three
Two
One
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is formed between oxygen and fluorine in OF2?
Covalent bond
Double bond
Metallic bond
Ionic bond
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs of electrons are found on the oxygen atom in OF2?
Four
Three
Two
One
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the effect of lone pairs on the molecular geometry of OF2?
They have no effect
They make the molecule linear
They cause the molecule to be bent
They make the molecule tetrahedral
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of OF2?
Linear
Bent
Trigonal planar
Tetrahedral
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do the fluorine atoms in OF2 spread out as far as possible?
To maximize the molecule's size
To minimize repulsion between bonding pairs
To maximize bonding
To minimize repulsion between lone pairs
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many lone pairs and bonding pairs are there in OF2?
Three lone pairs and one bonding pair
One lone pair and three bonding pairs
Four lone pairs and no bonding pairs
Two lone pairs and two bonding pairs
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of lone pairs in determining the shape of OF2?
They determine the bond length
They have no significance
They determine the bond angle
They influence the molecular geometry
Similar Resources on Wayground

6 questions
Understanding Molecular Polarity
Interactive video
•
9th - 10th Grade

8 questions
Steric Number and Molecular Geometry
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and Intermolecular Forces
Interactive video
•
10th - 12th Grade

11 questions
Understanding Molecular Geometry
Interactive video
•
9th - 10th Grade

6 questions
Covalent Bonds and Compounds
Interactive video
•
9th - 10th Grade

10 questions
Lewis Structures and Resonance in SO2
Interactive video
•
9th - 10th Grade

6 questions
Chemical Bonding and VSEPR Theory Quiz
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry of XF2
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade